

Representative values for pharmacokinetic and pharmacodynamics parameters were established on the basis of healthy subjects aged 45-64 years receiving 5 g idarucizumab (see Pharmacology: Pharmacokinetics and Pharmacodynamics under Actions). In these studies the doses of idarucizumab ranged from 20 mg to 8 g and the infusion times ranged from 5 minutes to 1 hour. The investigated population consisted of healthy subjects exhibiting specific population characteristics covering age, body weight, race, sex and renal impairment. Idarucizumab potently and specifically binds to dabigatran and its metabolites and neutralises their anticoagulant effect.Ĭlinical Trials: Three randomised, double-blind, placebo-controlled Phase I studies in 283 subjects (224 treated with idarucizumab) were conducted to assess the safety, efficacy, tolerability, pharmacokinetics and pharmacodynamics of idarucizumab, given alone or after administration of dabigatran etexilate.
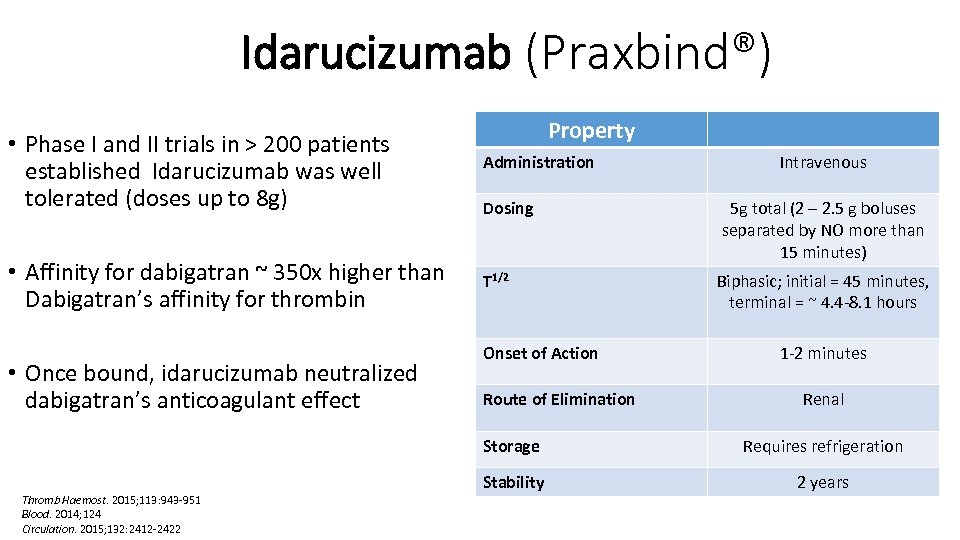
The idarucizumab-dabigatran complex is characterised a rapid on-rate and extremely slow off-rate resulting in a very stable complex. It is a humanized monoclonal antibody fragment (Fab) that binds to dabigatran with very high affinity, approximately 300-fold more potent than the binding affinity of dabigatran for thrombin. Pharmacology: Pharmacodynamics: Mode of action: Idarucizumab is a specific reversal agent for dabigatran. Pharmacotherapeutic Group: all other therapeutic products, antidotes.


 0 kommentar(er)
0 kommentar(er)
